Porifera-like Nickel Nanodendrite for Efficient Electrosynthesis of C-N Compounds from Carbon Dioxide and Nitrate Anions
Shivaraj B. Patil,1# Chang-Ru Lee,1# Swathi M. Gowdru,1# Chun-Chih Chang,2* Shu-Ting Chang,1 Yi-Chia Chen,1 Kuan-Chang Wu,1 Chia-Che Chang,1 Shu-Chih Haw,3 Di-Yan Wang1*
https://doi.org/10.1039/D3TA00438D
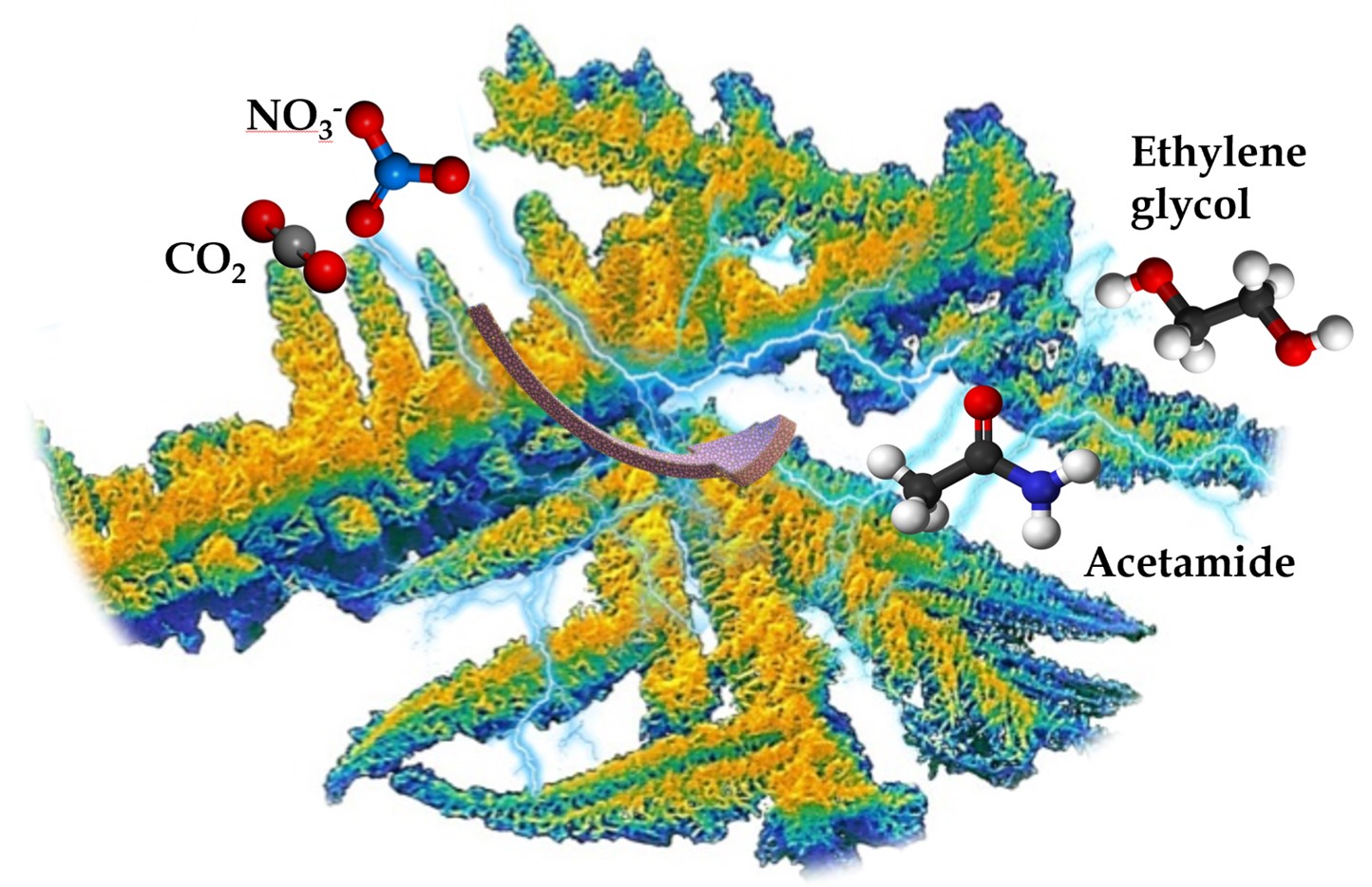
Generating high-energy compounds with heteroatomic bonds by using electrochemical reaction has attracted interest due to the high desire to achieve a net zero carbon state. In this dimension, heteroatomic compounds such as acetamide (CH3CONH2) was successfully produced along with formation of ethylene glycol and other C2+ compounds by integrating CO2RR with nitrate reduction reaction (NtRR). Highly porous nickel nanodendrites (p-Ni NDs) with porifera architecture was constructed by electrodeposition method and subsequent etching process. In the electrolyte of 0.05M KNO3 and 0.5 M KHCO3, p-Ni NDs can generate acetamide and ethylene glycol at the yield rate of 657 µg/h/cm2 and 640 µg/h/cm2 with FE of 23.2% and 18.0% under applying a voltage of -0.3 V vs RHE, respectively. 1H NMR was extensively used to detect and quantify the products. During the reaction, the surface of p-Ni NDs remains the metallic state which was confirmed by several X-ray spectroscopic techniques. Density functional theory (DFT) calculations revealed that *COHCOH(a) is the crucial intermediate in obtaining acetamide. Both experimental and theoretical experiments substantiate high activity of p-Ni NDs towards acetamide formation via C–N coupling.