Insights into Dynamic Molecular Intercalation Mechanism for Al-C Battery by Operando Synchrotron X-ray Techniques
Di-Yan Wanga , Shao-Ku Huangb, Hsiang-Ju Liaoa, Yu-Mei Chena, Sheng-Wen Wanga, Yu-Ting Kaoa, Ji-Yao Ana, Yi-Cheng Leea, Cheng-Hao Chuangc, Yu-Cheng Huangc, Ying-Rui Lud, Hong-Ji Lind, Hung-Lung Choue, Chun-Wei Chenb, Ying-Huang Laia and Chung-Li Dongc
https://doi.org/10.1016/j.carbon.2019.01.038
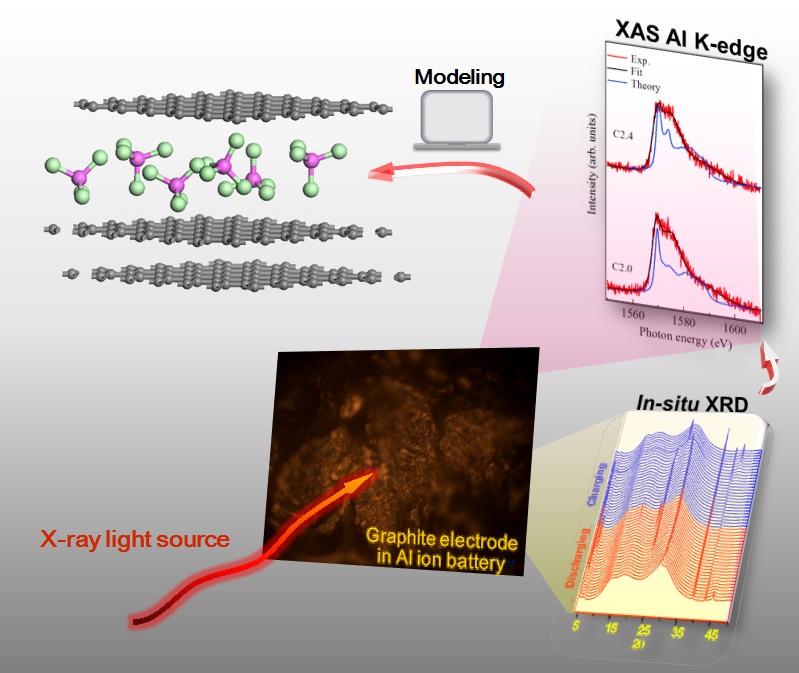
Recently, a novel rechargeable Al ion battery (AIB) with high performance has been developed. Owing to its low cost, high-rate capability, excellent cyclability, and non-flammability, AIB provides a safe alternative to many commercial batteries used in the energy storage system. To improve its performance, identifying molecular structure and arrangement of chloroaluminate anion (AlCl4−) intercalated in the graphite layer remains a great challenge. In this work, operando analysis of X-ray diffraction (XRD) measurement and X-ray absorption (XAS) spectroscopy were performed to investigate the intercalation of AlCl4− anion and related molecular arrangement in the graphite layers. The intercalated stage and intercalant gallery height of graphite cathode electrode during AIB battery operation were observed to be stage 3 and 9.22 Å, respectively. Furthermore, the spectral evolution from ex-situ XAS at the Al and Cl K-edge was associated with closely packed intercalation of AlCl4- molecules. With density functional theory (DFT) calculation, three simulated models of intercalated AlCl4‑ molecules in the graphite layer were revealed successfully, which explained possible molecular structures at different charging states. This analytical methodology paves the way for better understanding the structural transformation of molecular ions de-/ intercalation in graphite layers.