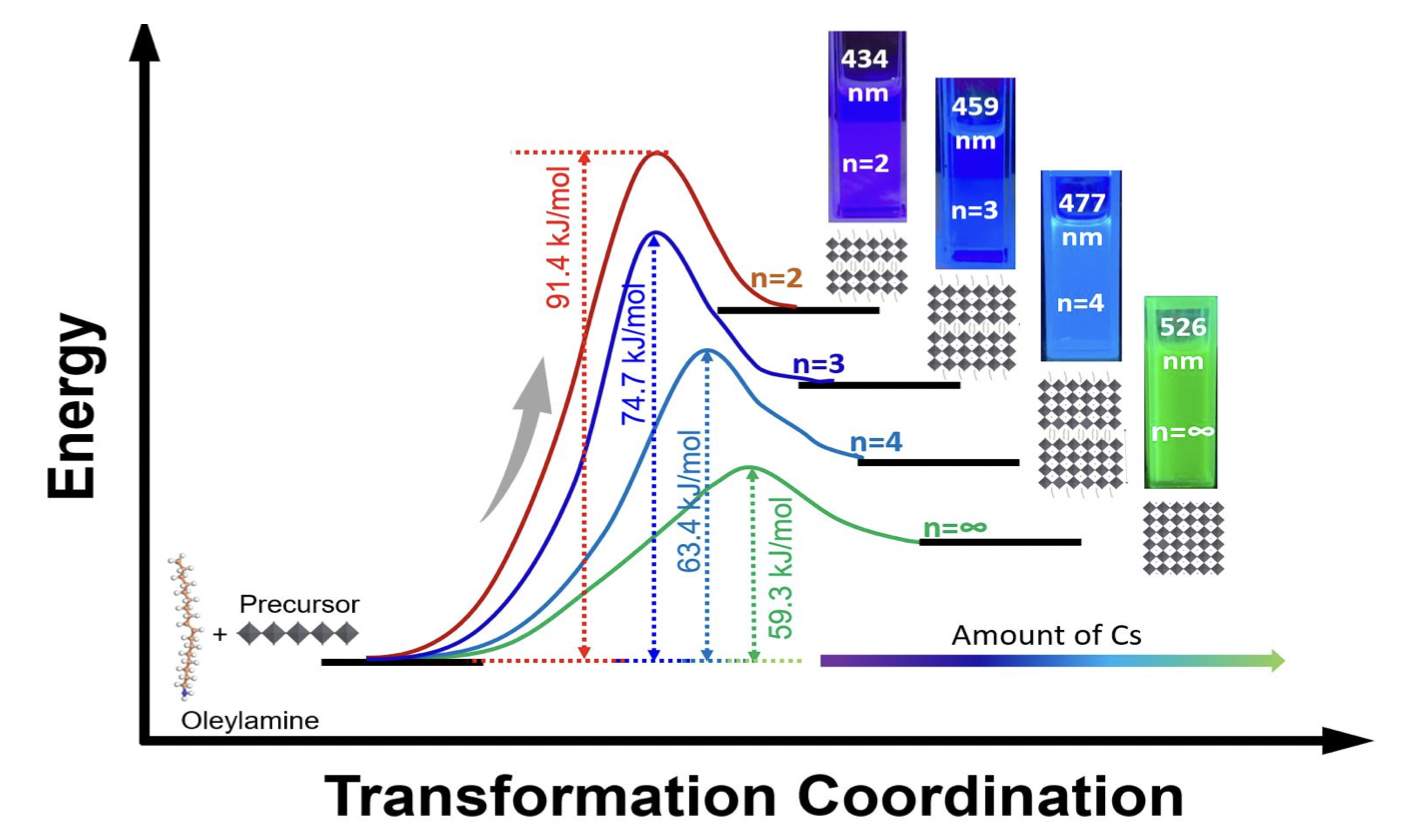
11 Jul. 2023, our work “Kinetic Studies of Oleylamine based 2D-Lead Bromide Perovskite with Controllable n-value by Sequential Addition of Cesium at Room Temperature” has been accepted by Journal of Physical Chemistry C
Invited Article in The Physical Chemistry of Perovskites VSI Virtual Special Issue.
Kinetic Studies of Oleylamine based 2D-Lead Bromide Perovskite with Controllable n-value by Sequential Addition of Cesium at Room Temperature
Anupriya Singh, Yi-Chia Chen, Kuan-Chang Wu, Shih-Mao Peng, Tsung-Rong Kuo, and Di-Yan Wang*
The outstanding properties of lead-based perovskite nanomaterials have led researchers to investigate the potential of their two-dimensional (2D) perovskite counterparts with the chemical formula A’2An+1BnX3n+1. Despite their advantages such as better stability and higher exciton binding energy, the synthesis of phase pure 2D perovskite nanomaterials remains challenge due to the fast nucleation process. Here, we demonstrate a simple and efficient method to synthesize 2D perovskite nanomaterials with phase pure specific n-values. The room temperature nucleation and growth of perovskites with specific n-values are controlled in a two-step synthesis method by the optimized amount of cesium. Our 2D perovskite nanomaterials with specific n-values exhibit high-purity photoluminescence (PL) peaks in the range from deep-blue to azure-blue emission color. X-ray diffraction (XRD) and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) were used to elucidate the kinetic reaction of oleylamine with PbBr2 and the effect of cesium addition in formation of 2D perovskite nanomaterials. In-situ photoluminescence studies were also performed to calculate the activation energy (Ea) of different specific n-value nanomaterials by using the Arrhenius equation. This study not only helps to visualize the effect of addition of Cs amount on the stacking of monolayers of [PbBr6]4-octahedrons, but also provides an easier way to control the n-value in 2D perovskite nanomaterials.
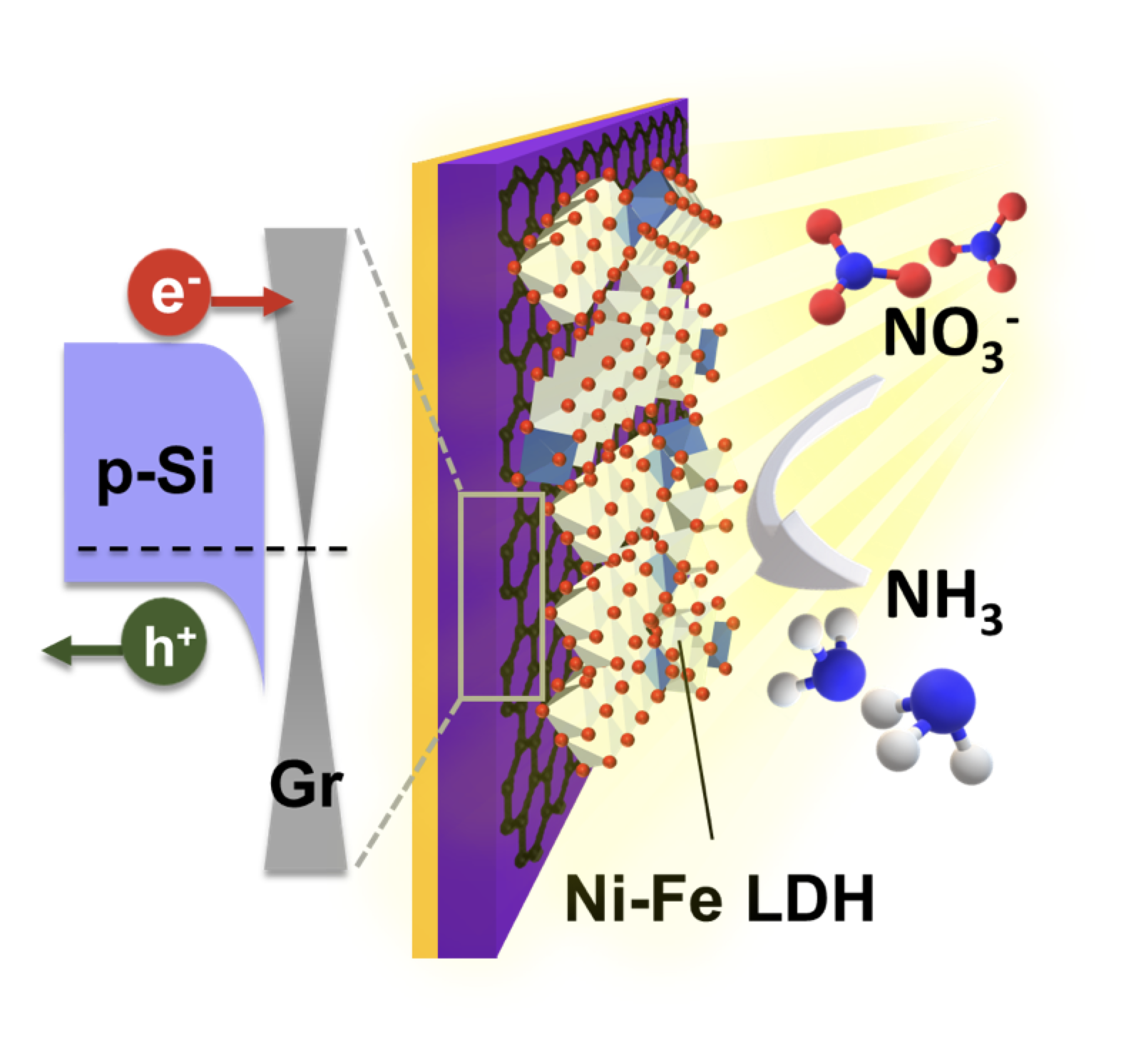
04 May 2023, our work “Efficient Ammonia Photosynthesis from Nitrate by Graphene/Si Schottky Junction Integrated with Ni-Fe LDH Catalyst” has been accepted on J. Mater. Chem. A
Efficient Ammonia Photosynthesis from Nitrate by Graphene/Si Schottky Junction Integrated with Ni-Fe LDH Catalyst
Chun-Hao Chiang, Yu-Ting Kao, Po-Hsien Wu, Ting-Ran Liu, Jia-Wei Lin, Po-Tuan Chen, Jr-Wen Lin, SHAN-CHIAO YANG, Hsuen-Li Chen, Shivaraj B. Patil, Di-Yan Wang* and Chun-Wei Chen*
https://doi.org/10.1039/D3TA01169K
This work presents the stable and efficient photoelectrochemical (PEC) nitrate-to-ammonia conversion through the facile integration of a graphene/Si Schottky junction and earth-abundant Ni-Fe layered double hydroxide (LDH). Efficient charge separation for photogenerated carriers and large photovoltage generation can be achieved resulting from the graphene/Si Schottky junction photocathode. Through the atomic layer of graphene, the direct growth of Ni-Fe LDH catalyst on the graphene/Si Schottky junction by electrodeposition provides excellent quality at the interfaces between the catalyst and photocathode. The Ni-Fe LDH/graphene/Si Schottky junction photocathode exhibits a promising and stable PEC conversion from nitrate to ammonia, with an optimal onset potential of 0.17 V vs. reversible hydrogen electrode (RHE), the largest saturated photocurrent density of -31.9 mA cm-2, and the highest Faradaic efficiency of 92.5% at 0.15 V vs. RHE. Combined with the several advantages of graphene, such as inherent chemical inertness, high optical transparency, and excellent conductivity, the integration of the semiconductor LDH catalyst on the graphene/Si Schottky junction platform provides an effective strategy to achieve stable and efficient PEC nitrate-to-ammonia conversion.