Fast Charge Transfer between Iodide Ions and Delocalized Electron System on the Graphite Surface for Boosting Hydrogen Production. just accepted by J. Mater. Chem. A
Shih-Mao Peng,1 Shivaraj B. Patil,1 Chun-Chih Chang,2 Shu-Ting Chang,1 Yi-Chia Chen,1 Kuan-Chang Wu,1 Wei-Nien Su,3,4* Bing Joe Hwang,4,5,6* Di-Yan Wang1*
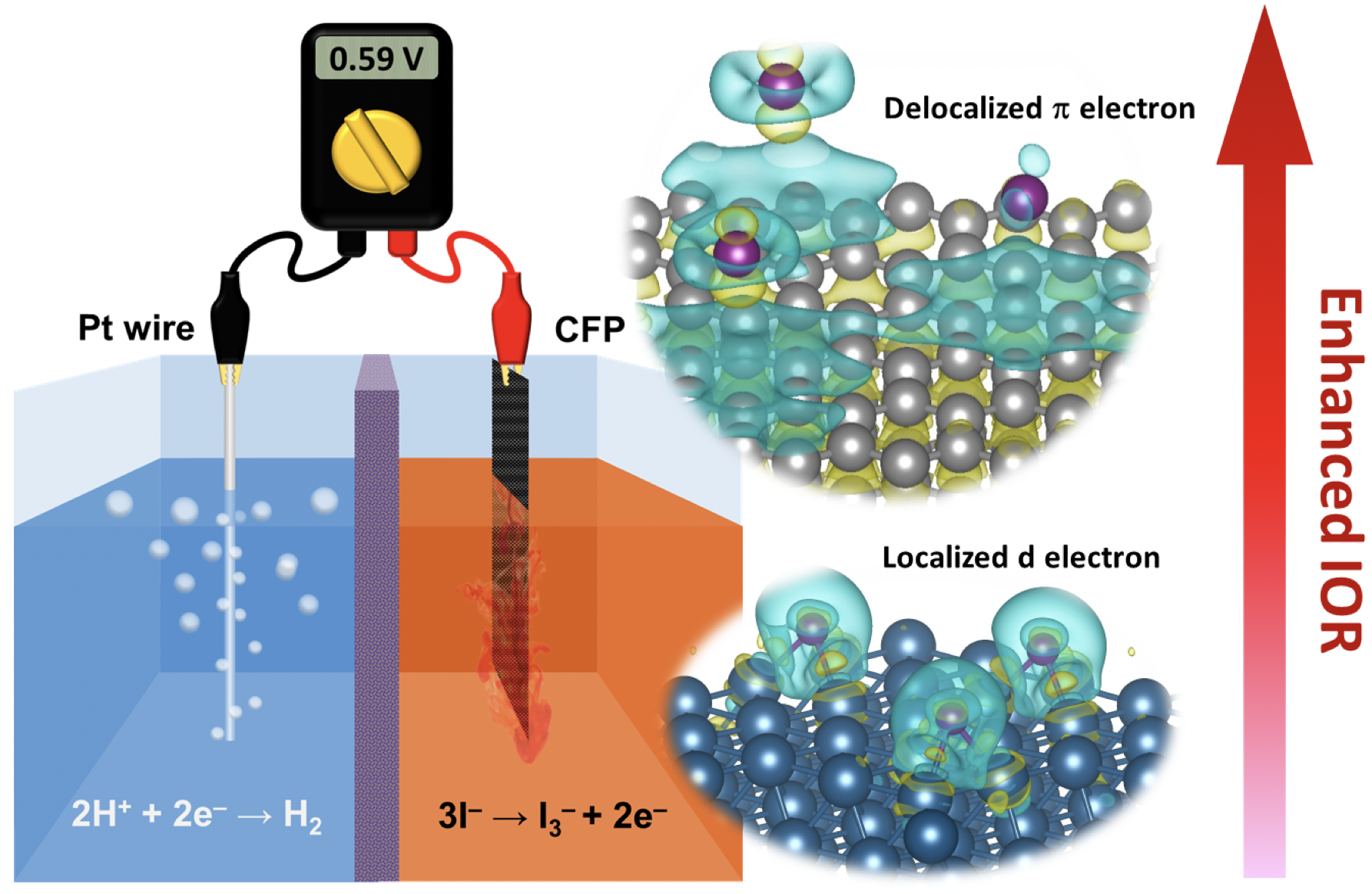
Developing new catalysts to reduce the energy barrier for hydrogen production from water splitting has been an important research topic. It is well-known that the water-splitting voltage is limited by a theoretical potential of 1.23 V of oxygen evolution reaction (OER). Recently, a new electrolytic method using iodide oxidation reaction (IOR) instead of OER has been proposed to produce valuable chemical iodine and hydrogen efficiently by a reduced operating voltage. In this work, we found that the graphite-based materials with a delocalized p electron system exhibited fast charge transfer with physically adsorbed iodide ions, thus revealing desirable IOR catalytic activity. The carbon fiber paper with a graphite structure was chosen as the catalyst and exhibited the onset potential of 0.54V (vs. RHE) for IOR, which is close to the standard potential of iodide oxidation. Also, the CFP demonstrated a minimum Tafel slope of 47.78 mV/dec in the electrochemical reaction with significant stability at the current density of 10 mA/cm2 for over 18 hours. Moreover, in a two-electrode system, a cell voltage of only 0.59 V was required to provide a current density of 10 mA/cm−2 for the IOR and hydrogen evolution reaction (HER). Based on energy costs, our HER and IOR system can reduce energy consumption by 65% compared to conventional OER-based water electrolysis. The overall results indicate that our findings pave a new route to facilitate the industrial application of hydrogen production via electrochemical reactions.