Near-infrared phototheranostic iron pyrite nanocrystals simultaneously induce dual cell death pathways via enhanced Fenton reactions in triplenegative breast cancer
Chunhua Zhao#, Zekun Liu#, Chia-Che Chang, Yi-Chia Chen, Qize Zhang, Xiao-Dong Zhang, Chrysafis Andreou, Jiadong Pang, Ze-Xian Liu *, Di-Yan Wang*, Moritz F.Kircher, Jiang Yang*
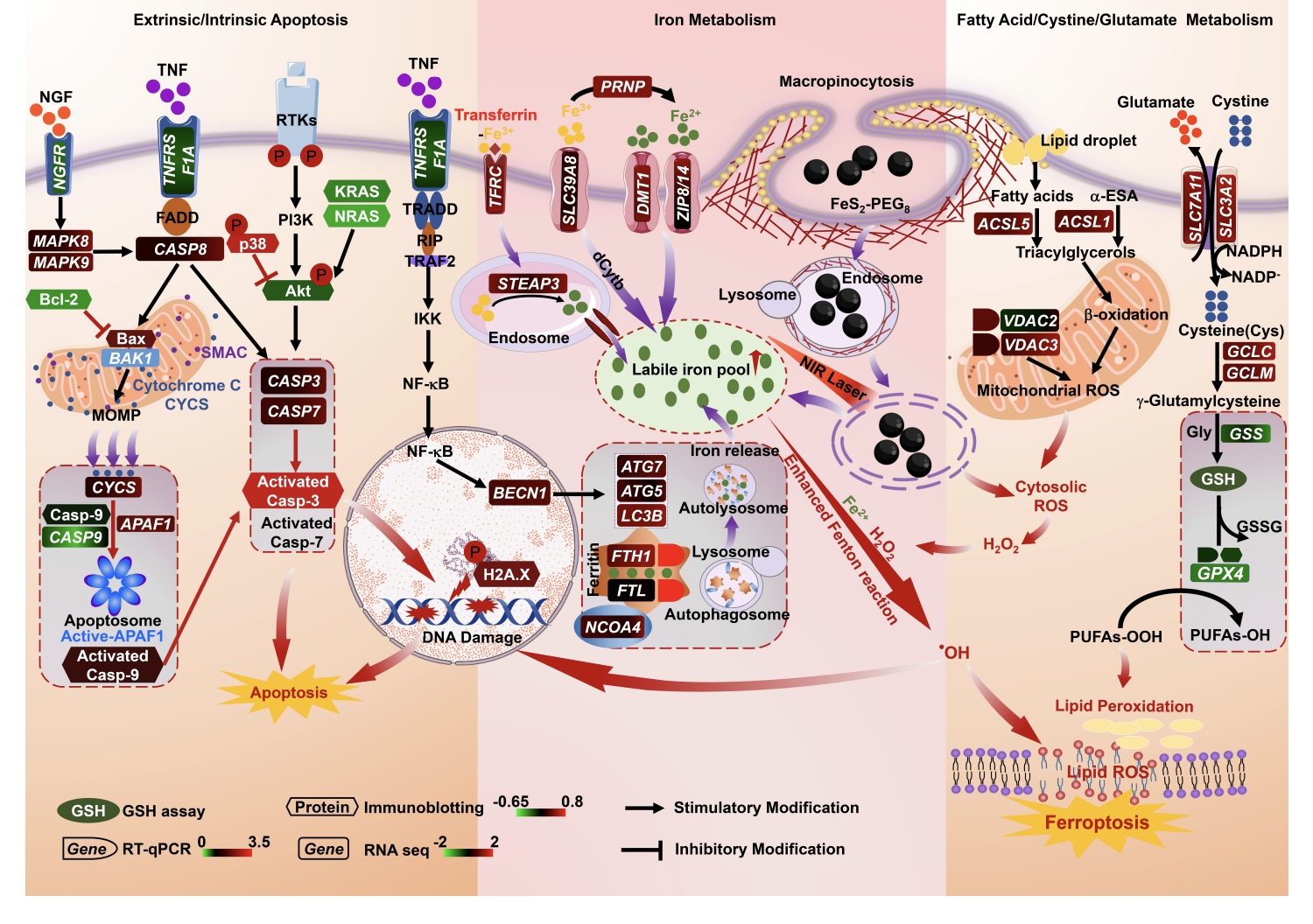
Triple-negative breast cancer (TNBC) is considered more aggressive with a poorer prognosis than other breast cancer subtypes. Through systemic bioinformatic analyses, we established the ferroptosis potential index (FPI) based on differentially expressed ferroptosis regulatory genes and found that TNBC has a higher FPI than non-TNBC in human breast cancer cell lines and tumor tissues. To exploit this finding, we developed biologically-amenable phototheranostic iron pyrite (FeS2) nanocrystals (NCs) that efficiently harness near-infrared (NIR) light, as in photovoltaics, for multispectral optoacoustic tomography (MSOT) and photothermal therapy. Upon NIR irradiation that thermodynamically enhances Fenton reactions, dual death pathways of apoptosis and ferroptosis are simultaneously induced in TNBC cells, comprehensively limiting cancer survival by regulating p53, FoxO, and HIF-1 signaling pathways and attenuating a series of metabolisms, including glutathione and amino acids. As a unitary phototheranostic agent with a safe toxicological profile, the nanocrystal represents an effective way to circumvent the lack of therapeutic targets and propensity of metastatic progression in TNBC and simplifies a streamlined workflow of cancer management with an integrated image-guided intervention.