Invited Article in The Physical Chemistry of Perovskites VSI Virtual Special Issue.
Kinetic Studies of Oleylamine based 2D-Lead Bromide Perovskite with Controllable n-value by Sequential Addition of Cesium at Room Temperature
Anupriya Singh, Yi-Chia Chen, Kuan-Chang Wu, Shih-Mao Peng, Tsung-Rong Kuo, and Di-Yan Wang*
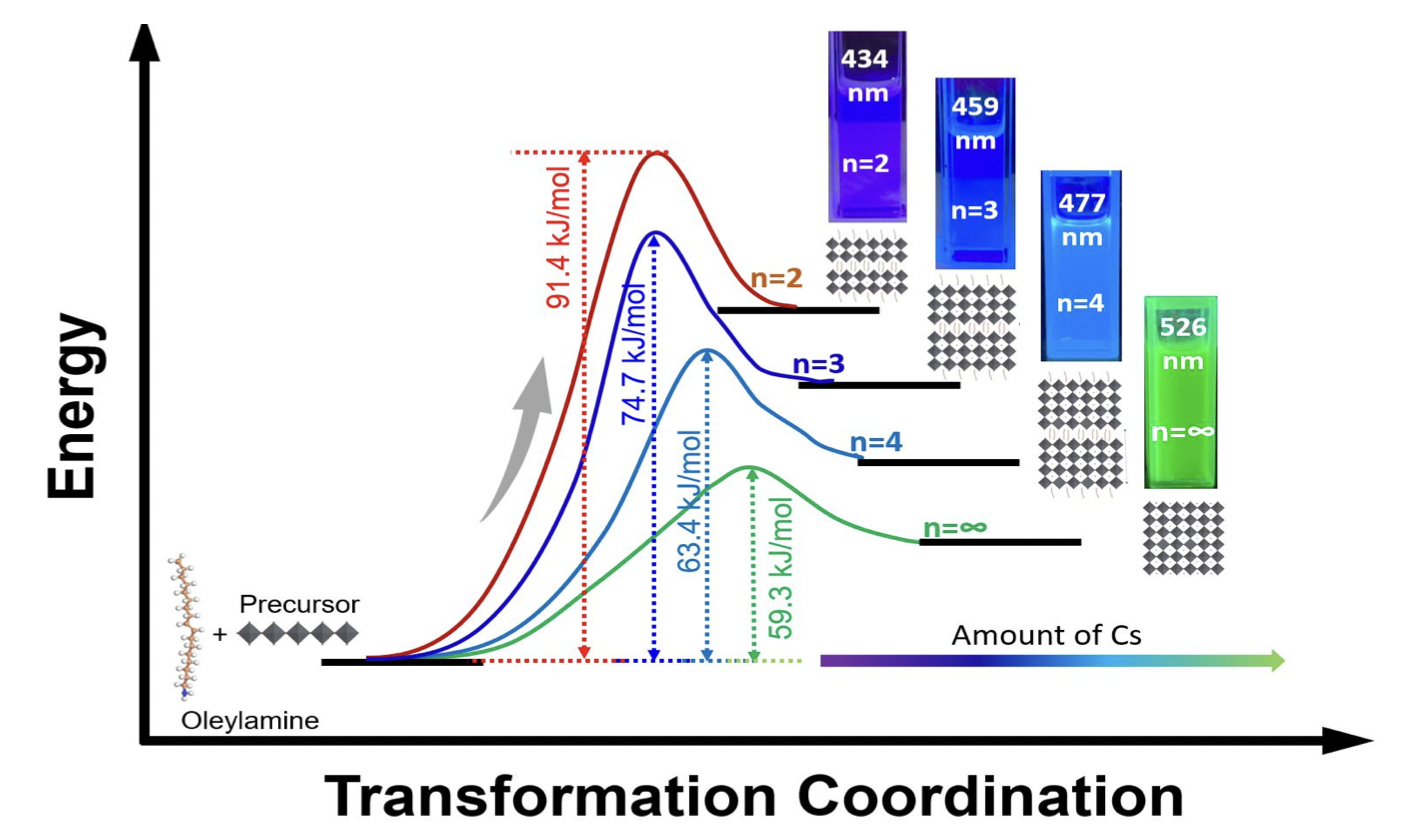
The outstanding properties of lead-based perovskite nanomaterials have led researchers to investigate the potential of their two-dimensional (2D) perovskite counterparts with the chemical formula A’2An+1BnX3n+1. Despite their advantages such as better stability and higher exciton binding energy, the synthesis of phase pure 2D perovskite nanomaterials remains challenge due to the fast nucleation process. Here, we demonstrate a simple and efficient method to synthesize 2D perovskite nanomaterials with phase pure specific n-values. The room temperature nucleation and growth of perovskites with specific n-values are controlled in a two-step synthesis method by the optimized amount of cesium. Our 2D perovskite nanomaterials with specific n-values exhibit high-purity photoluminescence (PL) peaks in the range from deep-blue to azure-blue emission color. X-ray diffraction (XRD) and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) were used to elucidate the kinetic reaction of oleylamine with PbBr2 and the effect of cesium addition in formation of 2D perovskite nanomaterials. In-situ photoluminescence studies were also performed to calculate the activation energy (Ea) of different specific n-value nanomaterials by using the Arrhenius equation. This study not only helps to visualize the effect of addition of Cs amount on the stacking of monolayers of [PbBr6]4-octahedrons, but also provides an easier way to control the n-value in 2D perovskite nanomaterials.