Electrocatalytic Reduction of NO3– to Ultrapure Ammonia on {200} Facet Dominant Cu Nanodendrites with High Conversion Faraday Efficiency
Shivaraj B. Patil,1Ting-Ran Liu,2 Hung-Lung Chou,3* Yu‐Bin Huang,1 Chia-Che Chang,1 Yi-Chia Chen,1 Ying-Sheng Lin,1 Hsin Li,1 Yi-Cheng Lee,1 Yuan Jay Chang,1 Ying-Huang Lai,1 Cheng-Yen Wen,2 Di-Yan Wang1*
https://doi.org/10.1021/acs.jpclett.1c02236
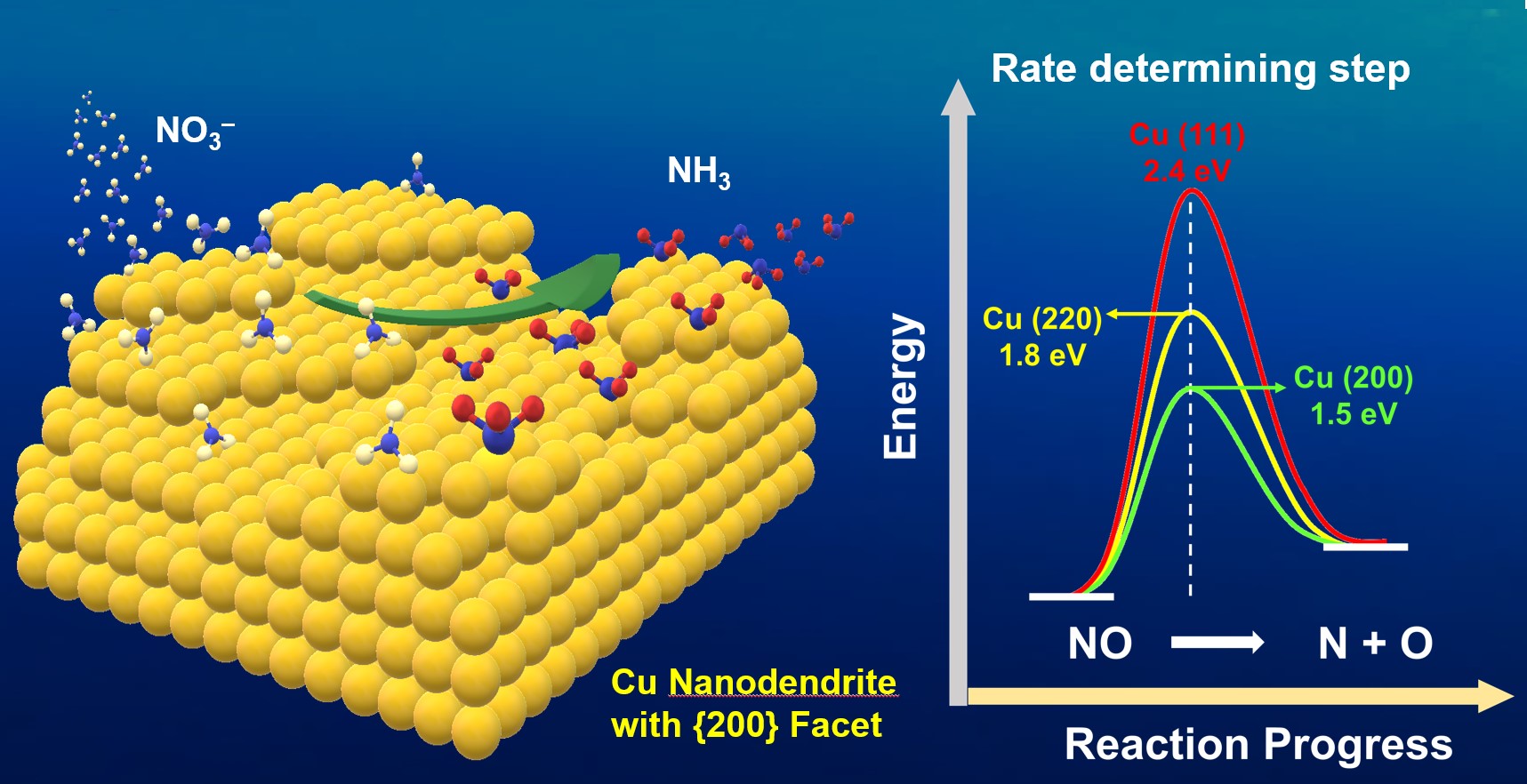
Nitrate (NO3–) reduction reaction (NtRR) is considered as a green alternative method for conventional way of NH3 synthesis (Haber-Bosch process), which is known as high energy consuming and large CO2 emitting process. Herein, the copper nanodendrites (Cu NDs) grown along with {200} facet as an efficient NtRR catalyst has been successfully fabricated and investigated. It exhibited high faradaic efficiency of 97% at low potential (-0.3 V vs RHE). Furthermore, the 15NO3ˉ isotope labelling method was utilized to confirm the formation of NH3. Both experimental and theoretical studies showed that NtRR on Cu metal nanostructure is a facet dependent process. Dissociation of NO bonding is supposed to be the rate determining step as NtRR is a spontaneously reductive and protonation process for all the different facets of Cu. Density functional theory (DFT) calculations revealed that Cu{200} and Cu{220} offer lower activation energy for dissociation of NO compared to that of Cu{111}.